The world constantly facing an increasing challenge of balancing between food security and the expanding human activities such as deforestation for agricultural, housing, and industrial activity. According to the United Nations, the global population had more than doubled in just 50 years reaching approximately 7.8 billion as of October 2020 compared to the 3.6 billion in 1970. Modern agriculture continues to evolve in order to accommodate the growing population, from which biotechnologies bring vital progress in terms of quality, quantity, and efficiency. Plant tissue culture is the magic cloning technique based on the theories of cell totipotency, that all plant cells have the ability to dedifferentiate, proliferate, and subsequently regenerate into mature plants under appropriate culture conditions.
The cloning technique is similar to that of a photocopy machine. Except other than producing same paper copies, the cloned plants are genetically identical to the parent plant. Normally only one plant grows from a viable seed, whereas plant tissue culture produces thousands of identical plants from a small piece of tissue of the parent plant. The rate of the production of plantlets is much faster than that of the conventional propagation through cutting and air-layering. Thus plant tissue culture is also referring to as fast-propagation or micropropagation.
Conventionally the selected hybrids or varieties with supreme traits such as higher yield are propagated through cuttings or air-layering to ensure genetic information that is identical to the parent plant. It’s important to avoid genetic variation that always comes with undesired or uninherited traits from the seed-germinated seedlings, which are the product of the combination of sexual gametes, pollens and ovaries via self- or cross-pollination. It’s a farmer’s nightmare to deal with a plantation that crops are at uneven height or have different flowering time and fruiting periods. Machine harvesting requires uniform and standardization, same in the horticulture industry in which the batches of the ornamental plants must be the same height and flowering period for display or arrangement in the landscape.
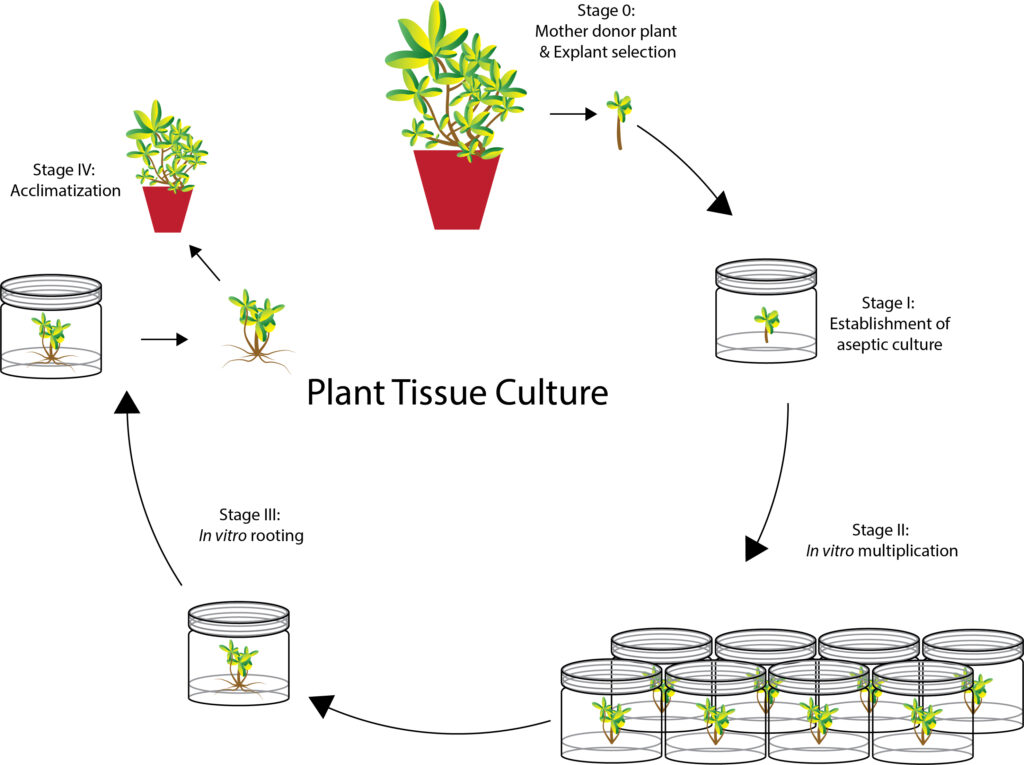
There are five steps to establish a complete process of plant tissue culture. The first step is the selection of elite mother plants that has the best germplasm, from which the large-scale multiplication of identical offerings will be produced. The following step is to establish an aseptic, or axenic culture. The in vitro, which means “in glass” condition has been applied to studies on living materials, that is performed outside the living organisms. For example, plant tissue cultures, cell homogenates, and subcellular fractions that are conducted from the explant, which is a part of the plant that is used as the starting material.
Theoretically, every part of the plant can be used as an explant, it could be meristematic tissue, somatic tissue, or a differentiated organ such as a leaf, stem, flower, seed, and root. However, practically the actively growing juvenile parts such as apical and axillary buds, young leaves, and hypocotyl of seed are often chosen as the explant for their active cell division. Organs such as roots, on the contrary, are less preferred due to their contact with soil or other substrates that have many microorganisms as well as bacteria and fungus. Also for a differentiated organ, it often starts with callus formation to dedifferentiate the cells before embryo induction, which is achieved through stimulation by auxins. The explants must be sterilized through surface sterilization to eliminate dirt, tiny bugs, bacteria, and fungal spores which may carry pathogens, airborne diseases by using surfactants such as alcohol (ethanol at 70 to 80 %) and other chemical reagents that have bleaching effects. The chemical reagents can be sodium hypochlorite, hydrogen peroxide, bromine water, and mercury chloride (highly biohazard). This step is conducted in an aseptic condition such as a working platform of a laminar flow hood or biosafety cabinet that equipped with high-efficiency particulate air (HEPA) filter.
After sterilization, the explant is cut into appropriate sizes by removing any damaged parts, and the fresh tissue is incubated onto a nutritional medium. The artificial nutrient medium is the formulation of nutrient compositions that is essential for plant growth and development, mainly inorganic nutrients such as macronutrients, micronutrients or trace elements, vitamins, and iron. Different explants and genotypes require different medium formulation. Commonly, the universal medium, which is known as Murashige and Skoog (1962) medium, is widely used in many species. However, there are many other options like woody plant medium, White’s medium, Gamborg B5 medium, and Hyponex medium that are designed for different plant species.
The carbon source sugar, as a supplement of photosynthetic products, is also an essential nutrient, it can be the monosaccharides (glucose, fructose, galactose) or disaccharides (sucrose, maltose, lactose). Besides, undefined organic supplements such as activated charcoal, mashed banana, coconut water, tomato, orange, and pineapple, extracts like beef, yeast, potato, or malt extracts can be added to promote tissue growth, organogenesis, or embryo formation. But the effects are case by case according to different genotypes, explants, and growing purpose. Plant growth regulator (PGR), or known as plant hormone or phytohormone, is utilized to regulate cell differentiation and optimize the growth of the plantlets. The most common PGRs used are cytokinin and auxin. The ratio of auxins and cytokinins play an important role in morphogenesis. A high ratio of auxins to cytokinins promotes embryogenesis, callus initiation, and root initiation, while a high ratio of cytokinins to auxin stimulates cell division, shoots formation, and cell differentiation. Sometimes antibiotics are added to reduce or suppress the growth of bacteria in the culture but may kill the plants as well. The nutrient media can be prepared by diluting the stock solutions of the nutrients into the working solution or simply dissolve the pre-mix media powder.
The growth medium is solidified by a gelling agent to make gelled media or called semi-solid media. The main characteristics of the gelling agents must be inert, be liquid when it is hot for dispensing into containers, autoclavable which withstand wet-heat sterilization process at 121 degrees Celsius for 15 minutes, that become semi-solid when it is cooled down to room temperature. It could be agar or gel with different textures in terms of firmness and clarity or transparency due to the extraction is originated from red algae (Gelidium amansii), bacterium (Pseudomonas elodea), or the husk of Plantago ovata seeds. The pH of a medium usually falls into the range between 5.7 to 5.8. The ions of the nutrients are readily available to the plants within the pH range mentioned. Different pH might destabilize the nutrients and make it less available to the nutrients.
After the establishment of axenic culture, the cultures enter the phase of shoot multiplication on the shoot induction medium (SIM) that is often added with cytokinin and a small amount of auxin to promote cell division and shoots formation. Adventitious shoots are induced and proliferated sometimes at exponential growth. After multiple rounds of subcultures of the newly formed in vitro shoot into new media, the multiplication rate can be achieved to satisfy the high demand of the market. Subsequently, the small shoots are subcultured onto root induction medium (RIM) that usually supplemented with auxin to promote root induction.
The last step is hardening or acclimatization. When the plantlets are ready to be taken out, the containers can be moved to a non-aseptic condition whereby the agar is washed off and then the rooted plantlets are planted into the soil or suitable substrates. The key to successful acclimatization is to reduce the humidity little by little in a chamber or greenhouse because the plants need time to adjust to a new environment. The physiological characterizations of in vitro plants subject to the saturated humidity conditions in a container are featured with significantly reduced numbers of stomata and cuticle layers, which are not suitable for growing in natural conditions. If plant them directly into an outdoor field, the sudden change in humidity and temperature will result in quick dehydration from transpiration, and the fragile cell wall will collapse which may kill the plants. The successfully acclimatized plantlets are normally planted in seedling trays or poly bags that are easy to transport around or ready to be planted in the field.
Plant biotechnology is the pivotal key to maintain and secure the food supply of the world. The advantage of plant tissue culture is its efficiency with high growth and multiplication rate, which is superior to propagation through seeds, cutting, or air-layering. Plant tissue culture is also the basic technique to crop improvement via genetic engineering, the asexual reproduction method allows the introduced or edited genes to be multiplied on a large scale. The meristem culture growing meristem tips at a faster speed than that of a pathogen spread to new cells enables the elimination of plant viruses and the propagation of virus-free plants. Besides, plant tissue culture is also useful in short-term and ex-situ conservation of the germplasm, production of synthetic seeds, and crop improvements other than GMO. (Next reading https://plantbiotechs.com/endless-possibilities-plant-tissue-culture/)
Further reading:
Carnivorous conservation. https://plantbiotechs.com/conservation-of-carnivorous-plants/
Endless possibilities – plant tissue culture. https://plantbiotechs.com/endless-possibilities-plant-tissue-culture/
Agriculture – Crop improvement. https://plantbiotechs.com/crop-improvement/