The largest flower in the world, Rafflesia (Rafflesiaceae), however, is from a small obligate endo-holoparasitic Asian plant genus. Although famous for its huge flowers, it’s a rare species and only lives in a small and special area that is high island endemism. This genus is classified as a member of Malpighiales, and native to tropical Southeast Asia, mainly found in Indonesia, Malaysia, and the Philippines. There are roughly 30 to 37 Rafflesia species that are currently recognized under the family of Rafflesiaceae, many of which are rare and threatened by habitat destruction, degradation, and fragmentation. About fourteen are found in Indonesia, thirteen in the Philippines, and around twelve in Malaysia. However, the numbers vary from year to year as some are considered extinct or some are newly under-discovered. Plant survey in the forest is an extremely challenging task, not to mention that the flowering period of Rafflesia sp. only lasts a few days. It has been reflected by the difficulties of sampling and data collecting.
Their flower size, rarity, as well as parasitic lifestyle, and associated highly specialized morphology make this genus valuable for biological research and germplasm conservation. Rafflesia has high island endemism: all but six species (R. arnoldi R. Br., B. cantleyi Solms, R. gadutensis Meijer, R. patma Blume, R. rochussenii Teijsm. & Binn., R. speciose Barcelona & Fernando) are endemic to Peninsula. Because Rafflesia plants and their Tetrastigma sp. hosts grow in various tropical rainforest ecosystems. The hosts appear to be relatively common and widespread. It is unlikely that the high island endemism results from very narrow environmental tolerances or host species with small distribution ranges. To date, there is no proof that Rafflesia sp. able to have parasitic-host interaction with other species than Tetrastigma sp. and no articles had established in vitro propagation.
Rafflesia is a dioecious species in which it has male and female plants separated. It has a high mortality rate, especially for the buds that are less than five centimeters in diameter, which grow on the ground. This is because it might die due to a lack of nutrients from the host, natural disasters, human or animal disturbances, insect infestation, trampling, predation, or precipitation (high humidity). When there is a sign of termites around or within the bud, this means that it had already dead as termites would not attack healthy growing buds. Other natural threats are due to the activity of the Rafflesia hunter for flower collection, logging and deforestation, and shifting cultivation. The estimated life cycle of Rafflesia is about three to four and a half years on average. This species is extra challenging to be studied as it has prolonged flower development after the bud initiation, which is around three to four years. Moreover, the rarity of the Rafflesia is partly due to the low density of the population.
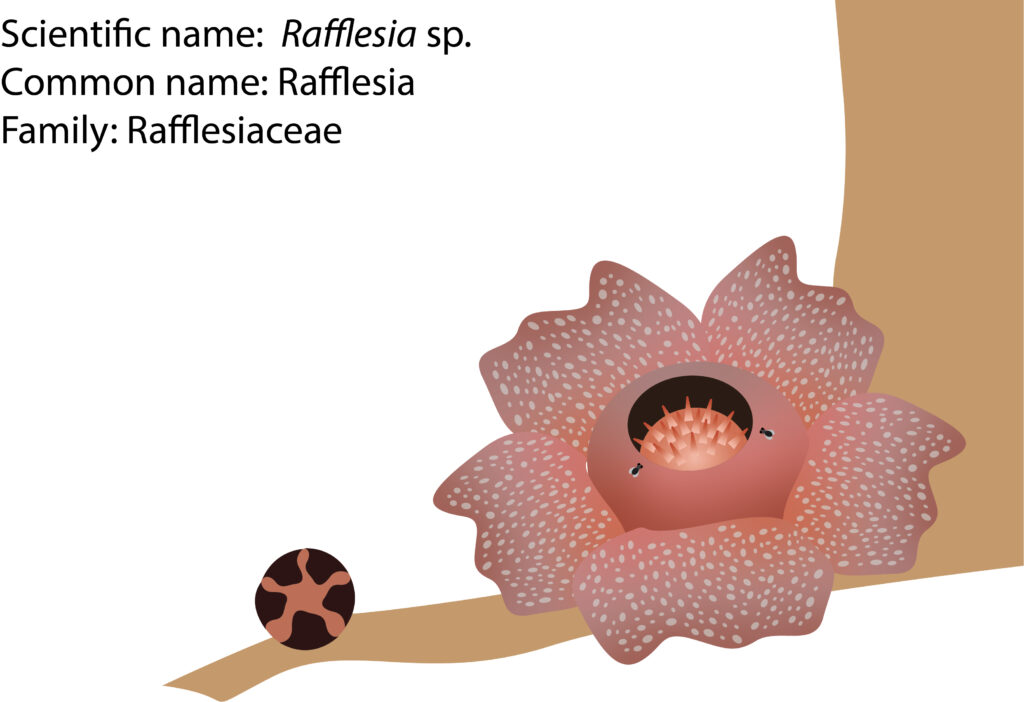
In general, Rafflesia has a floral diameter of about twenty centimeters attached to the vine of its specific host, Tetrastigma, through a connection called a haustorium. It is more likely that Rafflesia seeds are poorly dispersed between islands. This is because their life cycles are relatively short compared to other plants, ranging from three to five years. It takes about 2-3 years to develop the bud before flowering. Therefore, the low flowering incidence is proven with other difficulties for pollination and dispersal. To have successful pollination, there must be one male and a female flower bloom together at the same time. The primary pollinator is forest flies, namely, Chrysomya sp., Lucilia sp., and Hypopygiopsis sp. Rafflesia produces a stinky or rotten smell when it is bloom. It is believed that it had specialized in stink-smell-producing-flower as there are very little or no pollinators like bees and butterflies in the forest. Hence, when the smell attracts the flies, they walk around in the male flower, and from there, the pollen of the male flower is sticking onto their back. After that, the flies will visit the female flower. Thus, pollination and fertilization occurred. If there are two flowers of the same gender blooming simultaneously, no fertilization will happen.
The dispersal of seeds remains unknown and likely dispersed by animals who are into tiny seeds like wild pigs, ground squirrels, ants, termites, pangolin, and elephants. Some researchers concluded that small mammals most likely conduct the dispersal like adult tree shrew and adult plantain squirrel. On the other hand, small insects like ants are attracted by Rafflesia, elaiosome, which may help to carry the seeds away from the fruit to their nest. From there, the seeds germinate and infect the root or vine of the nearby Tetrastigma.
Besides, this parasitic plant also possesses active compounds that have beneficial biochemical properties to humans as it is rich in tannin, alkaloid, phenolic compounds such as catechin, proanthocyanidin, and phenolic acid. Traditionally, it is used in wound healing for its anti-microbial activity. It is also used for women to stop internal bleeding and shrink the womb after childbirth in Peninsular Malaysia. Furthermore, the bud was consumed by men as an energy drink or an aphrodisiac. However, due to the high concentration of tannin and phenols, it’s harmful when consumed in a large amount.
Despite the alarming situation of on the brink of extinction and various Ex-situ conservation methods have been actively explored to propagate Rafflesia and species conservation. The germination mechanism of the seeds on host plants is not clearly revealed and difficult to simulate. The test of seed germination in the laboratory using plant tissue culture has not been successful. So far, the only methods to conserve are to set up some fences to prevent tourists and small animals from destroying them and to perform grafting to relocate them to a “safer” place with more protection.
Since Rafflesia can only be found in the oldest lush tropical jungle in Malaysia and a few other places. Rafflesia azlanii is featured on the new ten-ringgit Malaysia banknote indigenous to Peninsular Malaysia, that is first discovered in the Royal Belum Forest Reserve of the state of Perak in the year 2003. None of the botanical gardens managed to display a living Rafflesia due to their short life cycle during blooming. So there are not many choices left for people who wish to witness their gigantic, beautiful yet bizarre blooming. Are you willing to pay a visit to the countries that have Rafflesia after the global pandemic is over?
Further reading:
| Abdulla, M. A., Ahmed, K. A., Ali, H. M., Noor, S. M., Ismail, S. (2009). Wound healing activities of Rafflesia hasseltii extract in rats. Journal of clinical biochemistry and nutrition, 45, 304-308. |
| Nickrent, D.L. (1997). The Parasitic Plant Connection. Available at: http://parasiticplants.siu.edu/ (Last accessed 1stDec,2019). |
| Meijer, W. (1997). Rafflesiaceae. Flora Males., Ser.113,1–42. |
| Hidayati, S.N., Meijer, W., Baskin, J.M., Walck, J.L. (2000). A contribution to the life history of the rare Indonesian holoparasite Rafflesia patma (Rafflesiaceae). Biotropica 32,408–414. |
| Nais, J. (2001). Rafflesia of the world. Sabah Parks, Kota Kinabalu. |
| Barcelona, J. F., Pelser, P. B., Balete, D. S., Co, L. L. (2009). Taxonomy, ecology, and conservation status of Philippine Rafflesia. Blumea, 54,77–93. |
| Mursidawati, S., Ngatari, N., Irawati, I., Cardinal, S., Kusumawati, R. (2015). Exsitu conservation of Rafflesia patma Blume (Rafflesiaceae): an endangered emblematic parasitic species from Indonesia. Sibbaldia, 13, 99–110 |
| Wicaksono, A., Mursidawati, S., Sukamto, L.A., Teixeirada Silva, J. A. (2016). Rafflesia spp.: propagation and conservation. Planta, 244,289–296. |
| Nikolov, L. A., Endress, P., Sugumaran, M., Sasirat, S., Vessabutr, S., Kramer, E. M., Davis, C.C. (2013). Developmental origins of the world’s largest flowers, Rafflesiaceae. Proc. Natl. Acad.Sci.U.S.A.110,18578–18583. |
| Xi, Z., Bradley, R., Wurdack, K., Wong, K. M., Sugumaran, M., Bomblies, K., Rest, J., Davis, C.C. (2012). Horizontal transfer of expressed genes in a parasitic flowering plant. BMCGenom.13,227. |
| Xi, Z., Wang, Y., Bradley, R. K., Sugumaran, M., Marx, C. J., Rest, J. S., Davis, C. C. (2013). Massive mitochondrial gene transfer in a parasitic flowering plant clade. PLoS Genet. 9e1003265. |
| Molina, J. E., Hazzouri, K. M., Nickrent, D. L., Geisler, M., Meyer, R. S., Pentony, M. M., Flowers, J. M., Pelser, P. B., Barcelona, J. F., Inovejas, S. A., Uy, I., Yuan, W., Wilkins, O., Michel,C.-I., Lock Lear, S., Concepcion, G. P., Purugganan, M.D. (2014). Possible loss of the chloroplast genome in the parasitic flowering plant Rafflesialagascae (Rafflesiaceae). Mol. Biol. Evol.31,793–803. |
| Barkman, T. J., Klooster, M. R., Gaddis, K. D., Franzone, B., Calhoun, S., Manickam, S., Vessabutr, S., Sasirat, S., Davis, C. C. (2017). Reading between the vines: hosts as islands for extreme holoparasitic plants. Am. J. Bot.104,1382–1389 |
| Nikolov, L. A., Davis, C. C. (2017). The big, the bad, and the beautiful: biology of the world’s largest flowers. J. Syst. Evol. 55,516–524. |
| Twyford, A. D. (2017). New insights into the population biology of endoparasitic Rafflesiaceae. Am. J. Bot. 104,1433–1436. |
| Ng, S. -M., Lee, X.- W., Mat-Isa, M. -N., Aizat-Juhari, M. A., Adam, J. H., Mohamed, R., Wan, K.-L., Firdaus-Raih, M.,2018.Comparative analysis of nucleus-encoded plastid-targeting proteins in Rafflesia cantleyi against photosynthetic and non-photosynthetic representatives reveals orthologous systems with potentially divergent functions. Sci. Rep.8,17258. |
| Wee, S. L., Tan, S. B., Jürgens, A. (2018).Pollinator specialization in the enigmatic Rafflesia cantleyi:a true carrion flower with species-specific and sex-biased blow fly pollinators. Phytochem. 153, 120–128. |
| Hidayati, S. N., Walck, J. L. (2016). A review of the biology of Rafflesia: what do we know and what’s next? Bul. Kebun Raya19,67–78. |
| Pelser, P. B., Nickrent, D. L., Gemmill, C. E. C., Barcelona, J. F. (2017). Genetic diversity and structure in the Philippine Rafflesia lagascae complex (Rafflesiaceae) inform its taxonomic delimitation and conservation. Syst. Bot.42,543–553. |
| Barcelona, J. F., Pelser, P. B., Balete, D. S., Co, L. L. (2009). Taxonomy, ecology, and conservation status of Philippine Rafflesia. Blumea 54,77–93. |
| Barcelona, J. F., Fernando, E. S., Nickrent, D. L., Balete, D. S., Pelser, P. B. (2011). An amended description of Rafflesia Leonardi and a revised key to Philippine Rafflesia (Rafflesiaceae). Phytotaxa 24,11–18. |
| Pelser, P. B., Nickrent, D. L., Barcelona, J. F. (2016). Untanglinga vine and its parasite: host specificity of Philippine Rafflesia (Rafflesiaceae).Taxon 65,739–758. |
| Pelser, P. B., Nickrent, D. L., Barcelona, J.F., 2018. A conservation genetic study of Rafflesia speciosa (Rafflesiaceae, Philippines): patterns of genetic diversity and differentiation within and between islands. Blumea 63, 93-101. |
| Surya, N. W., & Idris, M. (2011). A preliminary study on in vitro seed germination and rooted callus formation of Tetrastigma rafflesiae (Vitaceae). Gard Bull Sing, 63(1), 499-505. |